
Today we are happy presenting in our Speaker’s Corner an article of our Chinese colleagues Liu Shen about the implementation of a drug patent linkage system in China which allows – similar to the Hatch-Waxman Act in the US – an early patent dispute resolution between innovative drug developers and generic drug manufacturers before generic drug approval:
Dr. Yuan Zhang, Dr. Stephen Zou
Discussion on establishing the patent linkage system in China has been carried out for years. With the recent revision of Chinese Patent Law, such system was introduced as a principled provision and has come into force since 01 June 2021. In July 2021, the drug regulatory department (NMPA), the patent office (CNIPA) and the Supreme Court issued implementation measures and judicial interpretation to specify operational details, representing the settlement of this long-discussed system. In this article, an overview is provided on the infrastructure of this brand-new system by giving answers to frequently asked questions.
I. General Questions
1. What is “linked” under the patent linkage system?
Under the patent linkage system, the drug regulatory process is linked with patent protection, which means, in principle, a drug would not be approved if it falls within the scope of an effective patent listed on the patent information platform.
2. What is the role of patent linkage in the pharmaceutical industry?
Patent linkage intends to balance the interests of the public and the brand-name drug companies.
On the one hand, it provides an approach for the brand-name drug companies to assert their rights before the marketing of an alleged product, thus to protect the innovator’s interest and encourage the initiative of innovation.
On the other hand, it gives the generic drug company more certainty on their marketing strategy and prevents damages that may be put on the company’s side if a commercialized product is determined infringing. Further, patent linkage also provides the generic drug company an approach to advance the commercialization of generic drugs by challenging the relevant patent or making a statement of non-relevance.
As a result, under a well-functioning patent linkage system, more effective drugs would pop up thanks to the protection of innovators’ interest, and the patients would have access to drugs with a more reasonable price due to the advanced commercialization of generic drugs.
3. Where does the patent linkage stand in the drug life cycle?
The patent linkage stands in between of the research and commercialization, meaning it would only take over when the generic drug company has finished the clinical trial and submitted the application, but not obtains the approval yet.
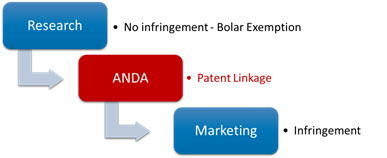
4. Which departments are involved in the patent linkage?
Patent linkage would involve the coordination of the NMPA, the CNIPA, and the court:
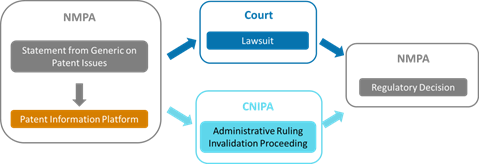
II. Patent Information Platform
1. Where does the generic drug company go to look for relevant patent information?
The NMPA has established a patent information platform to list the patents relevant to the approved drugs, which is the basis for the generic drug company to make patent statement and for the marketing authorization holders (MAH) or the patentee to assert the rights under the patent linkage system.
2. Which entity is eligible for listing the patent information on the platform?
It is the MAH to list patent information on the platform. If the MAH is not the patentee, an authorization from the patentee is to be provided.
3. Is there a deadline for listing the patent information?
Yes, the deadline is 30 days from the date of obtaining the Certificate of Pharmaceutical Product (COPP). If there is any change on the patent information, the information should be updated within 30 days from the date when the changes take place.
4. Is it necessary to have the relevant drug approved for the patent listing?
Yes. Having the drug approved in China is a pre-requirement for relevant patents to be listed since the drug approval information is to be provided for the listing.
5. What types of patents are eligible to be listed the platform?
The eligible patents are summarized in the following table:
| Chemical Drugs | Biologics | Traditional Chinese Medicines (TCM) | |
| Active Ingredients | Compound | Sequence | Extract |
| Formulation | √ | X | √ |
| Medical Use | √ | √ | √ |
The patents of intermediates, metabolites, crystalline forms, manufacture processes, and detecting methods are not eligible.
6. Are the pending patent applications eligible for listing?
No. The letters patent are required to be submitted for the listing. Therefore, a pending patent application cannot be listed on the platform.
7. What information and document is necessary for the listing?
The information to be provided for the registration involves three aspects: drug, patent, and MAH, which are summarized in the following table.
| Drug | Patent | MAH |
| Drug type (chemical, biological or TCM) Drug Name Dosage Form Strength Drug approval number | Patent Number Patent Title Patentee Licensee (if licensed Issued Date Letters Patent | MAH’s relationship with the patentee (being patentee itself, or being the licensee of an exclusive/ non-exclusive/ sole license) Contact Information |
To be noted, the contact information needs to be accurate, since it would become the basis of judicial service during the patent linkage litigation.
8. Can the MAH choose to input the contact information of an agency or attorney on the platform?
So far there is no regulation saying “no”, and we’ve seen some companies have done so. This is more convenient and efficient when the patentee is abroad and the MAH is a joint venture company only partly owned by the patentee.
9. If the MAH is a licensee, is the license agreement required to be recorded in CNIPA?
So far there is no regulation requiring the MAH to record or provide the license agreement, while we would suggest having the agreements ready just in case.
10. Is the listing mandatory?
No. The MAH could choose not to list their patents. However, having the relevant patent listed on the platform is the basis for the MAH or patentee to assert their rights under the patent linkage system. Therefore, if the patent is not listed, the MAH or the patentee can only assert the rights after the commercialization of the generic drug.
11. Is there any opposition proceeding against the listed patent information?
It is only generally stipulated that relevant opposition against the listed patents should be verified and recorded, with no specific regulation on the proceeding, e.g., how to raise an opposition and who to examine. Therefore, it is currently unclear how the opposition system would work and we may have to wait to see how the NMPA would handle such issue in the near future.
III. Generic Drug Company’s Patent Statement
1. When should the generic drug applicant submit the patent statement?
The patent statement should be submitted along with the generic drug application.
2. How many types of statement are specified under the patent linkage system and how would the NMPA handle each type of statement?
The statement will be categorized into four types:
| Type | Statement Content | NMPA’s reaction |
| I | No relevant patent is found on the platform. | Approve the product if technical requirements are met. |
| II | The relevant patent is no longer effective or the generic drug company has been granted a license on it. | Approve the product if technical requirements are met. |
| III | The generic drug company commits not marketing the generic drug until the relevant patent expires. | Put the generic drug application on hold until the relevant patent expires. |
| IV | 4.1 The relevant patent should be declared invalid; 4.2 The generic drug would not fall within the patent scope. | The patentee or the MAH could take action within 45 days from the publication of the Type IV patent statement. If a decision is made in favour of the patentee, the NMPA will put the generic drug application on hold until the relevant patent expires. |
3. Would there be a regulatory waiting period if the patentee or the MAH make response to the Type IV statement?
For chemical drugs, the patentee or the MAH’s action would trigger a 9-month waiting period , during which the NMPA would not approve the generic drug.
No waiting period is applicable for biologics and TCM . Therefore, there is no delay of regulatory approval for such two type of medicines, and the regulatory proceeding of the product would only be put on hold if a decision is made before NMPA’s approval.
4. How would the waiting period be early ended?
The waiting period would be ended if (1) there is an effective judgment from the court or an administrative ruling from the patent office confirming the generic drug would not fall within the patent scope; or (2) the relevant patent has been declared invalid.
5. Is the court or the CNIPA required to make a decision within the waiting period?
There is no such requirement. If no final decision is available at the end of the waiting period, the NMPA will continue the regulatory proceeding, and it is possible for the generic drug to be approved before the issue of a decision on the patent dispute.
6. What is needed to submit a Type IV statement?
Along with the Type IV statement, the generic drug company should also submit the claim chart between the generic drug and the relevant claims, as well as the relevant technical materials.
7. How would the patentee or the MAH be notified of the Type IV statement?
The generic drug company is responsible to notify the MAH of the Type IV statement within 10 working days from the acceptance of the generic drug application. The MAH’s contact information on the platform is the basis of the notification. If the MAH is not the patentee, the MAH should then notify the patentee.
8. How to coordinate among the court, CNIPA, and NMPA?
The coordination among various departments lies on the shoulders of the patentee, the MAH, and the generic drug company.
Specifically, first, as mentioned above, the Type IV statement would be notified by the generic drug company to the MAH, along with the claim chart and relevant technical materials. Then, if the MAH or the patentee takes action, they should notify the NMPA and the generic drug company within 15 working days of the acceptance of the case. Once a decision is made, any one of the patentee, the MAH, and the generic drug applicant could notify the NMPA of the result within 10 working days of receiving the decision. If it is an administrative ruling, the CNIPA would also copy the NMPA of the decision.
9. Is there any marketing exclusivity for the generic drug applicant that succeeds the patent challenge?
Yes, the entity who first succeeds the patent challenge and first obtains the marketing authorization would be granted a 12-month marketing exclusivity, starting from the drug approval date, during which the NMPA would not approve the same type of products.
10. What is the definition of succeeding the patent challenge?
To be the first successful challenger, the applicant needs to: (1) submit a Type IV statement; and (2) successfully invalidate the relevant patent.
11. Is there any exception during the 12-month exclusivity?
Yes, a co-challenger who also successfully challenges the patent would become an exception, meaning the same type of product from such co-challenger could be approved during the 12-month exclusivity. However, so far there is no clear definition on the meaning of co-challenger.
IV. Dispute Resolution
1. What are the approaches for the patentee or MAH to take action against the generic drug application?
The patentee or MAH could either file for a lawsuit before the court (judicial approach) or apply for an administrative ruling before the patent office (administrative approach).
2. What is the nature of a patent linkage litigation/ruling?
It is a confirmation procedure rather than an infringement litigation/ruling, meaning, in principle, the court or the patent office need only to decide how to construct the claims and whether the generic drug falls within the patent scope.
3. Is there any approach for the generic drug applicant to take action under the patent linkage system?
If the MAH or the patentee does not take action within the 45-day period, the generic drug applicant could ask for a lawsuit or an administrative ruling to confirm its product not falling within the scope of the relevant patent.
4. Would the doctrine of equivalent (DOE) be considered during the patent linkage litigation/ruling?
From legal perspective, the DOE should be involved. As mentioned above, the patent linkage litigation/ruling is a confirmative proceeding mainly focusing on claim construction, for which both the literal and equivalent infringement should be considered according to the SPC’s judicial interpretation in 2009.
5. What is the same and difference between the judicial and administrative approaches?
The following table compares the judicial and administrative approaches:
| Judicial | Administrative | |
| Eligible Party to take action | Patentee, MAH, Licensee (Brand); Generic Drug Applicant (Generic) |
|
| Timing | Brand: 45-day from publishing the patent statement Generic: If the Brand takes no action within the 45-day period |
|
| Jurisdiction | Beijing IP Court | CNIPA |
| Burden of Proof | Brand: patent, generic drug information, Type IV statement and its basis;
Generic: technical material of generic drug |
|
| Can the proceeding be suspended by invalidation? | In principle, no | CNIPA may choose not to suspend the ruling. |
| Can the proceeding coincide with another approach? | Not affected by a parallel administrative proceeding. | The case would not be accepted if there is a lawsuit. |
| Decision acceptable by NMPA | Effective judgment | Administrative ruling, not necessarily to be effective |
6. What are the pros and cons of the judicial and administrative approaches?
The administrative approach could be more efficient since an administrative ruling would be enough for the NMPA to make a decision, and the ruling does not necessarily to be effective. On the contrary, the judgment from judicial approach needs to be effective, meaning the proceeding would take longer if there is an appeal.
The advantages of judicial approach lies in that, an effective judgment issued by the court would be final. On the other hand, an administrative ruling may need to go through judicial review, meaning it could potentially be reverted by the court. Meanwhile, the patent linkage litigation could potentially be less time-consuming than a typical infringement litigation due to the confirmative nature, making it less uncompetitive with the administrative counterpart in terms of efficiency. Of course, this depends on how the Beijing IP court would handle such type of litigation.
A
The above outlines the infrastructure of China’s patent linkage system. Under this brand-new system, both the brand-name drug company and generic drug company need to adjust their policy to accommodate. For brand-name drug companies, the first priority is to sort out their IP assets, so as to determine the patent(s) to be listed, identify the potential weakness, and have itself prepared for challenges from competitors. For generic drug companies, more effort needs to be put on the IP due diligence to identify relevant patent in advance and perform patentability analysis to determine the corresponding strategy. Coordination between the timing for raising challenges and marketing products is also paramount in obtaining the 12-month marketing exclusivity.
A
A
Established in 1993, Liu Shen & Associates is a leading intellectual property law firm in China. With over 200 professionals, we provide comprehensive and integrated services covering all aspects of intellectual property, including patent prosecution, enforcement, IP transaction, and related litigation and administrative proceedings. For more information, please contact Dr. Yuan Zhang (yuanzhang@liu-shen.com) and Dr. Stephen Zou (zlzou@liu-shen.com).




