In March last year, the much-awaited decision of the Enlarged Board of Appeal (EBA), G 2/21, issued on the topic of “plausibility”, a catchword much applied in the context of the required quality of disclosure and evidence in the application as filed for a contested purported technical effect to be further supported by post-published evidence. The question may arise in the context of inventive step (Article 56 EPC) or sufficiency of disclosure (Article 83 EPC), in case the effect is expressly claimed.
When the written decision of G 2/21 finally was published there was some disillusionment regarding the concreteness of the provided guidelines because the decision remains rather abstract.
It is not surprising that the guidance given by the Technical Boards applying the criteria given in G 2/21 was now equally much-awaited. While the case law will continue to develop, there are meanwhile several decisions that shed light on the interpretation. In particular the decision T 0116/18 (Board 3.3.02) on the case underlying G 2/21 is now available.
T 0116/18: Standard under inventive step – the purported effect is not claimed
This decision relates only to inventive step (the purported technical effect is not specified in the claim) and gives a very detailed guidance for the application of the criteria for the allowance of post-published evidence in this context.
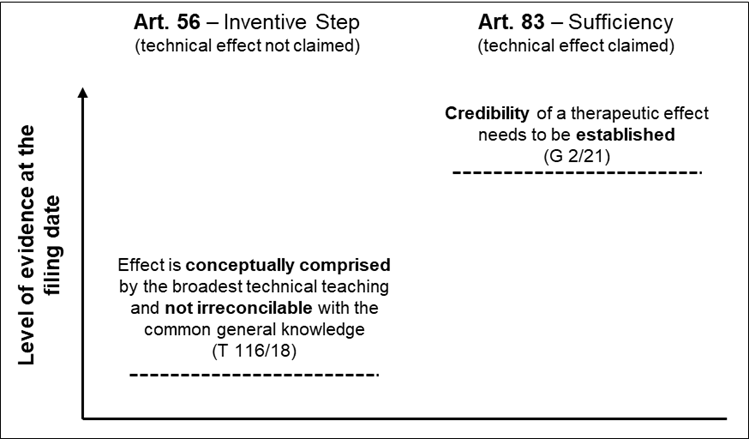
The Board applies a two-fold test derived from Headnote II of G 2/21, namely whether the purported effect would be i) “encompassed by the technical teaching” and ii) “embodied by the same originally disclosed invention”. It concludes that neither a verbatim statement nor experimental prove of the effect is required. Referring to national decisions on the “Apixaban case”, the Board expressly rejected the standard of plausibility applied by the UK Supreme Court and the requirement of a positive verbal statement of the effect in the application as filed by the Dutch Court of Appeal. Rather, in the context of prong i), it suffices that the purported technical effect together with the claimed subject-matter is conceptually comprised by the broadest technical teaching of the application as filed. And in the context of prong ii), a question to be answered is whether the effect is irreconcilable with the common general knowledge taken into account the application as filed. Based on this level of evidence the Board concluded in this case that the purported effect is in fact (i) encompassed by the technical teaching and (ii) embodied by the same originally disclosed invention. Without going into all the details of the underlying case, we conclude that the reasoning provides quite a lot of flexibility for applicants or patentees to use post-published evidence as long as the effect is within the general concept disclosed and does not raise a disbelieve in the sense of what could be considered as not “ab initio implausible” or now summarized as “irreconcilable with the common general knowledge”. The EPO practice leans toward the very old German approach: G 2/21 cites a still valid decision form the German Federal Court of Justice from 1972, X ZB 2/71 – Imidazoline that generously allows post-published evidence to substantiate inventive step.
Most other decisions citing G 2/21 rendered in the context of inventive step (the effect is not claimed) meanwhile seem equally generous in this respect, although not all apply the two-fold test. See T 0873/21 (3.3.07), T 0728/21 (3.3.07), T 1445/21 (3.3.07), T 1989/19 (3.3.02), T 0681/21 (3.3.06; regarding an auxiliary request), T 0885/21 (3.3.02), and T 1329/21 (3.3.07). Only two decisions, T 0258/21 (3.3.07) and T 0887/21 (3.3.04), and T 0681/21 regarding the main request did not accept the purported technical effect in accordance with G 2/21.
G2/21: Different standard under sufficiency – the purported effect is claimed
For us an important further and now rather clear learning from G 2/21 is the higher standard to be applied on the credibility of an effect when it relates to sufficiency of disclosure. While the concrete questions to be answered by the EBA arising from the underlying case were in the context of inventive step, the standard for such an effect under sufficiency of disclosure (the purported technical effect is specified in the claim) was also considered. The guidelines for sufficiency are now only in the body of the reasoning and not in the order providing the binding answers to the questions. It can nevertheless be assumed that those guidelines will be applied by the Technical Boards.
The EBA summarizes in paragraph 77 of G 2/21 that the scope of reliance on post-published evidence for sufficiency is much narrower. The EBA states, albeit only in the context of therapeutic effects, that a lack of proof or at least credible disclosure in the application as filed cannot be remedied with post-published evidence. This appears to be the old standard of “ab initio plausibility”.
In any event, looking at the guidelines for inventive step and how these are now applied and the guidelines for sufficiency of disclosure side by side, the level of evidence required at the filing date clearly differs. While an effect must be credible for sufficiency of disclosure, the standard is lower under inventive step in that the effect must only be conceptually comprised by the broadest technical teaching and not be irreconcilable with the common general knowledge (like the former “ab initio implausible” standard):